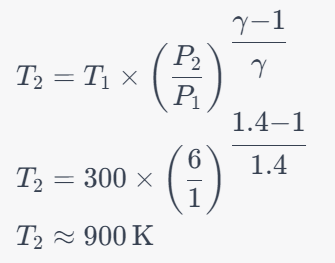In the last article, I have shared about unit of refrigeration, in this article you will learn about Bell Coleman Cycle– Processes, COP Formula, PV and TS Diagram, MCQ’s SSC JE, GATE.
What is Bell Coleman Cycle?
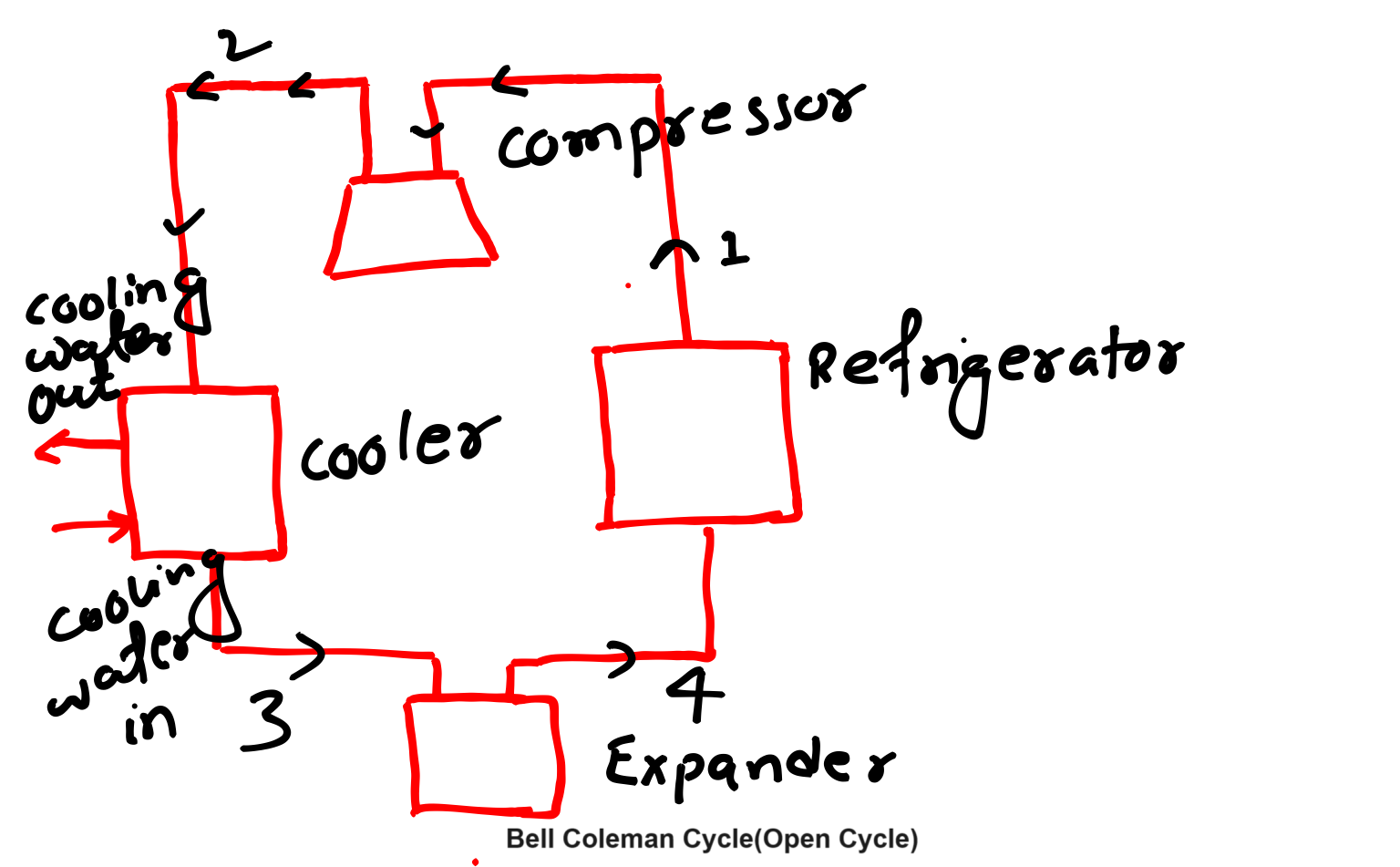
Other popular name of Bell Coleman cycle is reversed Brayton cycle as well as Reversed Joule Cycle, we can also say that it is a modified version of reversed Carnot cycle. This cycle is used in air refrigeration systems and cryogenic applications.
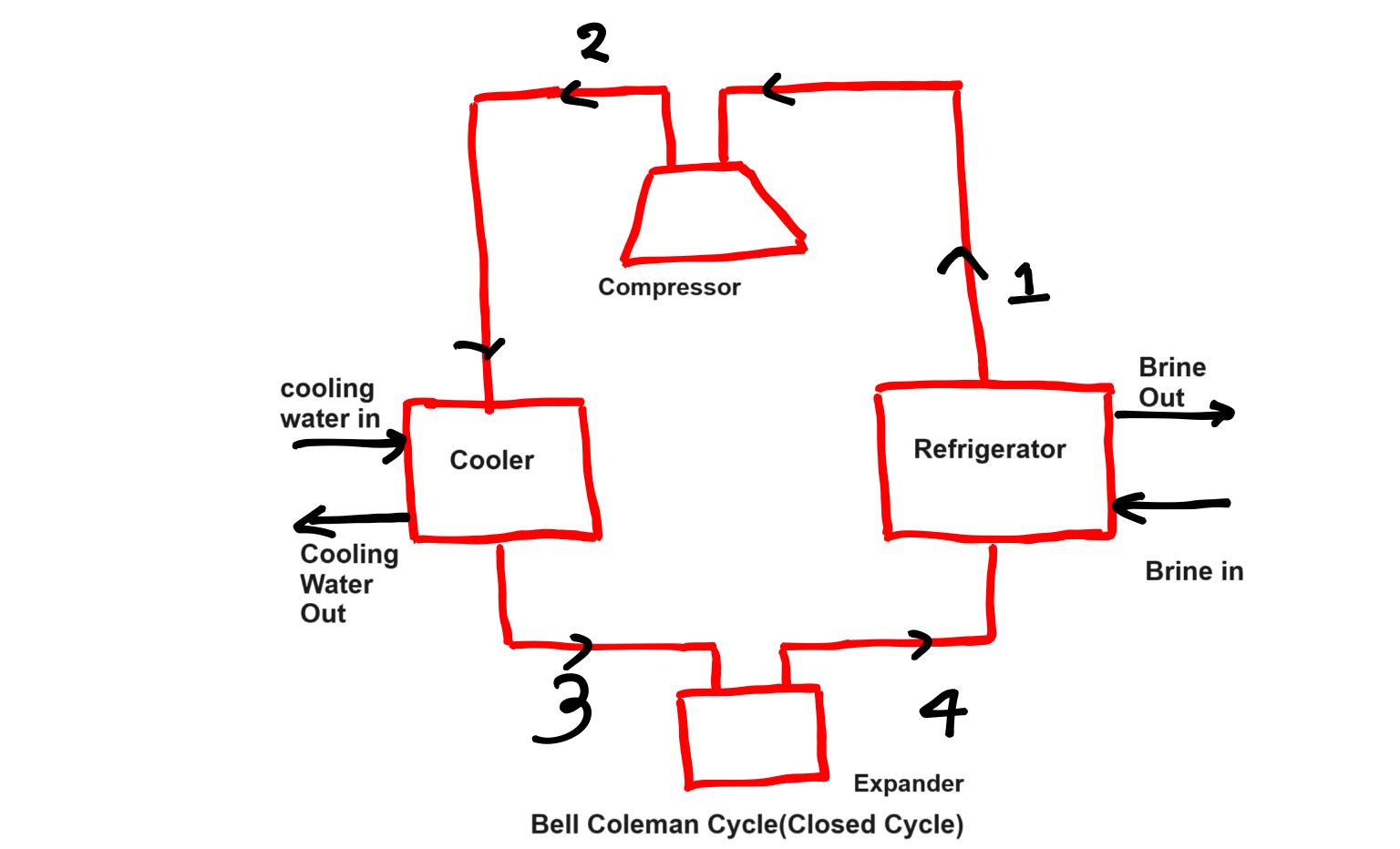
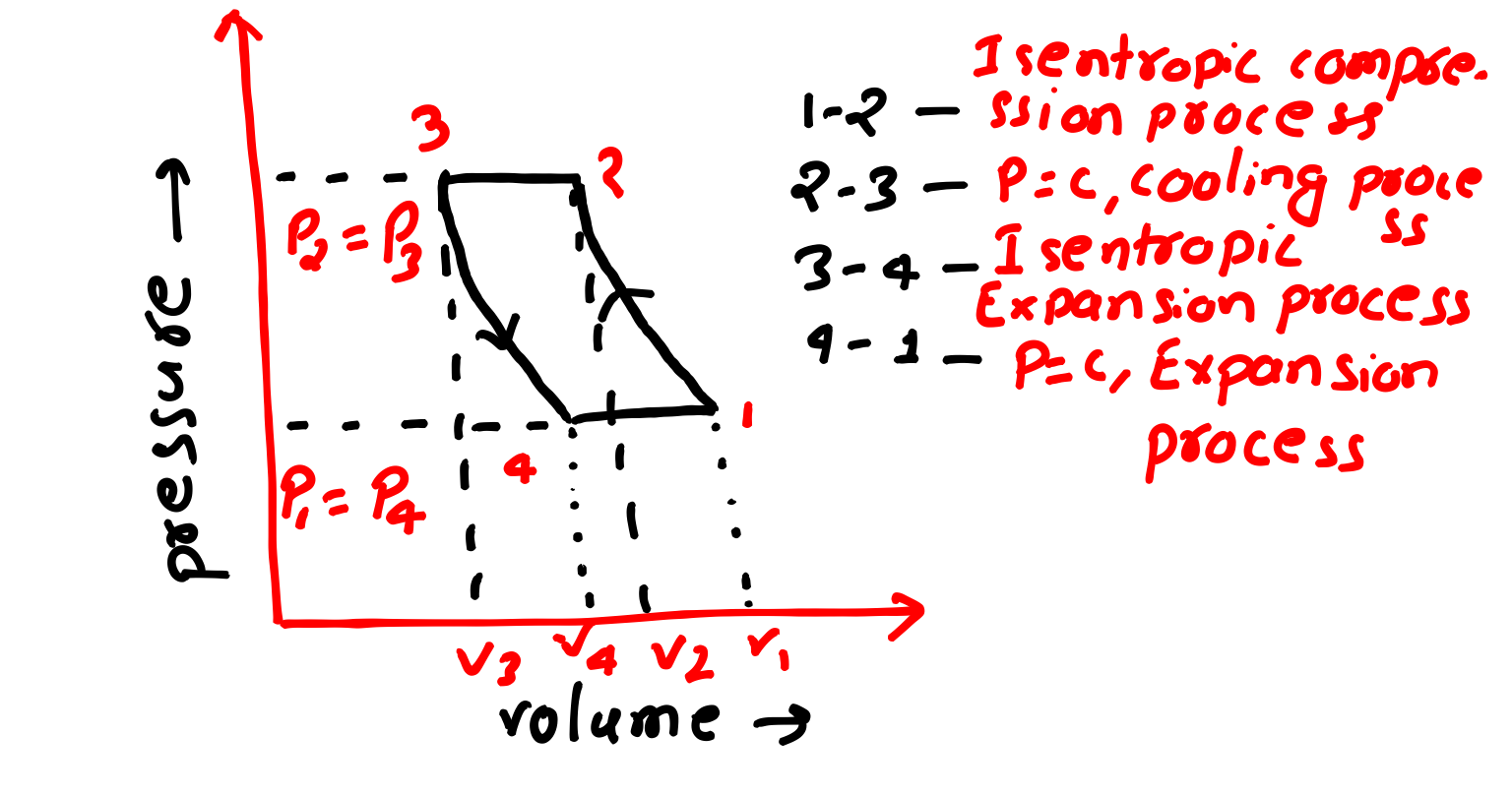
This refrigeration cycle having four processes those will discuss, diagram of Bell Coleman cycle is below-
Working Process of Bell Coleman Cycle-
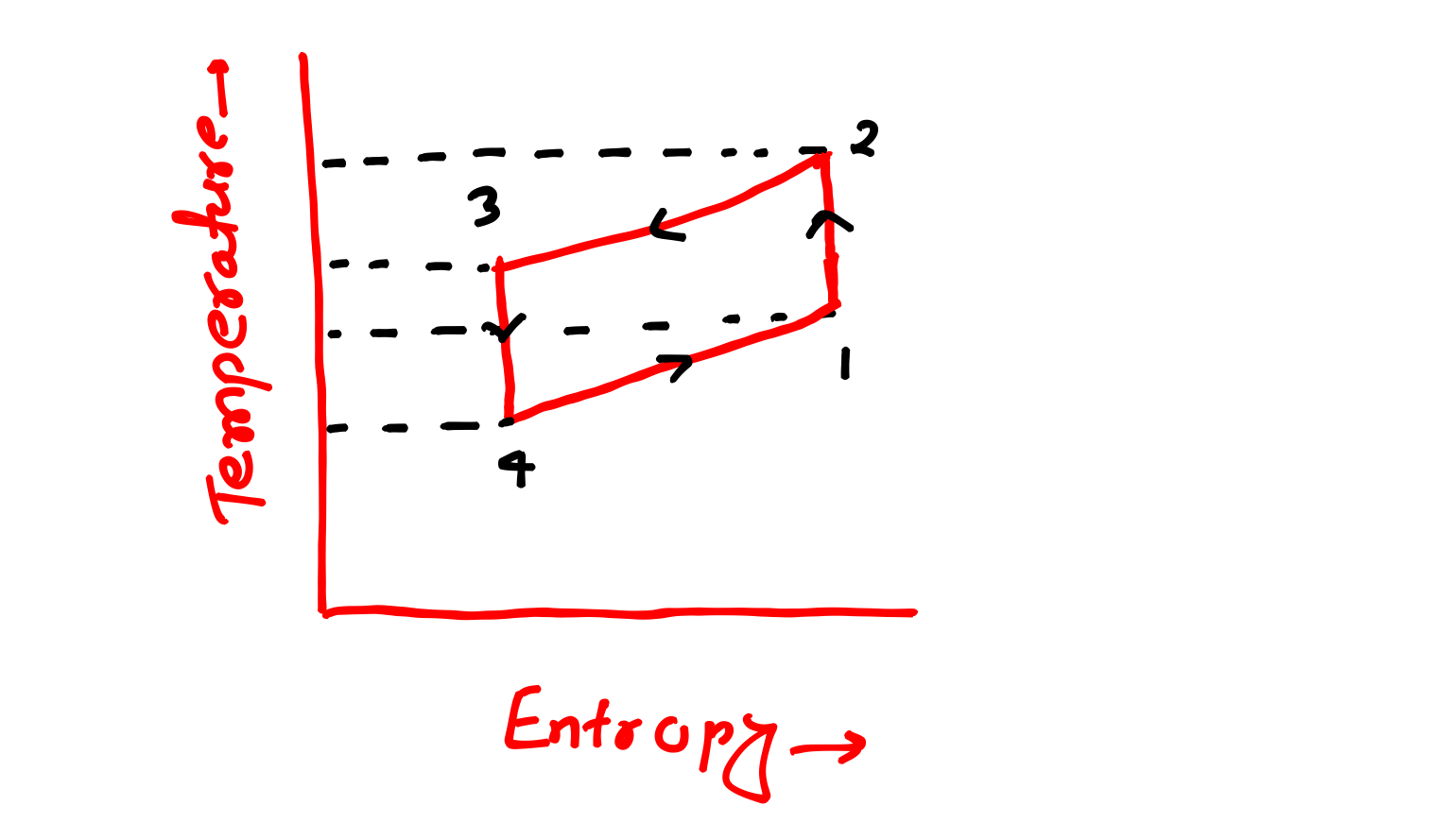
Lets understand all processes of Bell Coleman cycle, for proper understand of these processes try to watch out PV and TS diagram of this cycle both are given below.
Isentropic Compression Process-
In this process(1 to 2) no heat is absorbed nor heat is rejected, in this case entropy is constant, pressure and temperature increases but specific volume decreases which we can easily understand from PV and TS Diagram as well.
Constant Pressure Cooling Process-
In this process(2 to 3), now we got high pressure and temperature air from compressor which will pass through cooler, pressure constant but temperature and specific volume decreases.
In this process heat is rejected by air.
Mathematically, we can write
Q2-3 = Cp(T2 – T3)
Isentropic Expansion Process-
In this process( 3 to 4), after air passing through cooler now in this process it will pass through expander, in this process pressure and temperature both decreases by maintaining entropy as constant.
Because it is an isentropic process which also means reversible adiabatic process means no heat absorbed or no heat is rejected.
Constant Pressure Expansion Process-
In this process(4 to 1), we got cold air from expander now in this process air will pass through cooling portion of the refrigerator, pressure is constant but temperature increases but specific volume will increases. In this process heat is absorbed from the system.
Mathematically,
Q4-1 = Cp(T1 – T4)
Derivation of Bell Coleman Cycle-
Work done during the cycle per kg of air-
= Heat rejected – Heat absorbed
= Cp(T2 – T3) – Cp(T1 – T4)
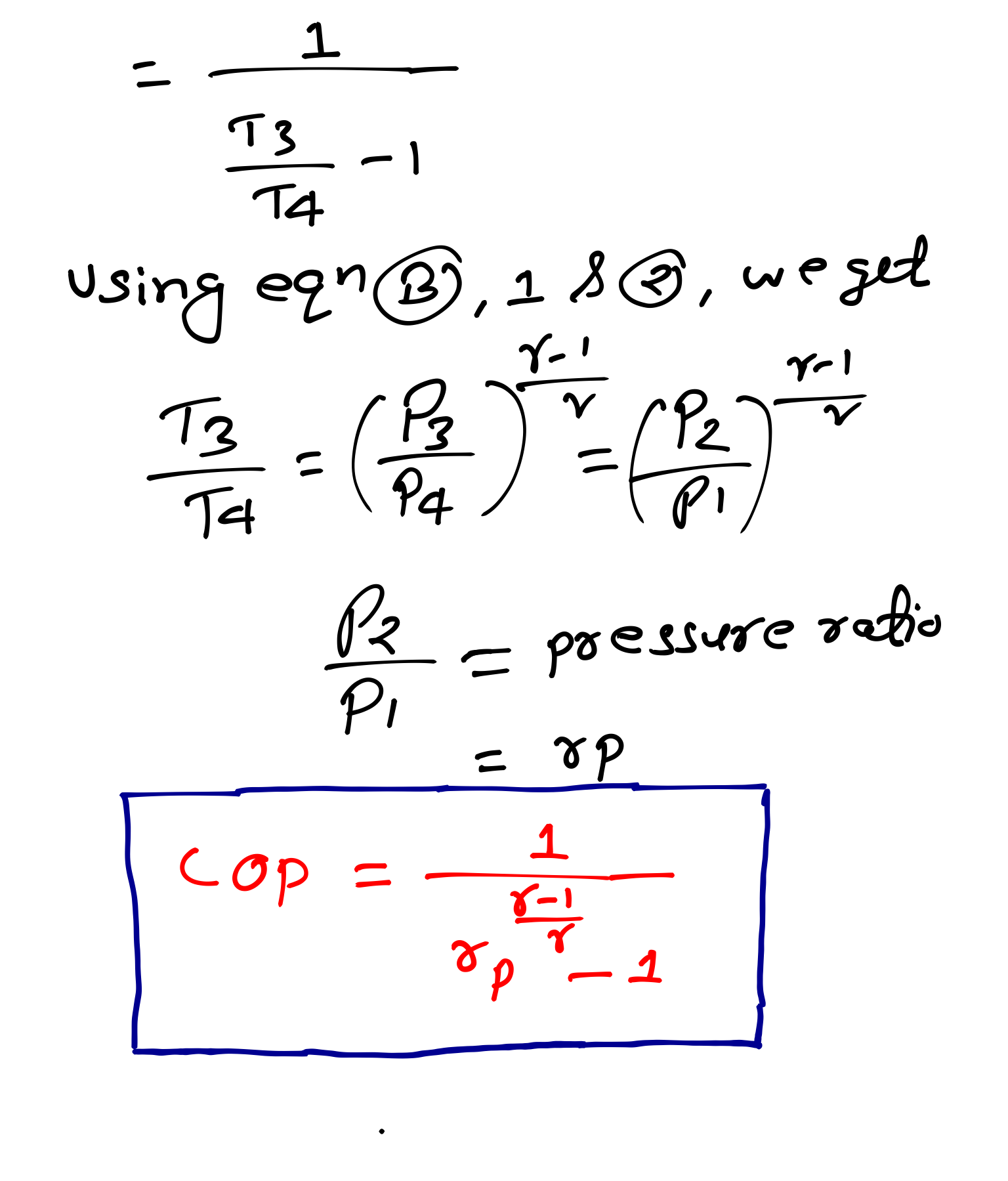
COP(Coefficient of Performance) = Heat Absorbed/Work Done

PV and TS Diagram of Bell Coleman Cycle-
MCQs of Bell Coleman Cycle-
In this section of this article, you will get mcqs with solution, so lets go for it.
Question 1: In the Bell Coleman refrigeration cycle, the working substance is:
a. Air
b. Water
c. Ammonia
d. Carbon dioxide
Solution: Answer: a. Air
Question 2: What is the primary purpose of the intercooler in the Bell Coleman cycle?
a. To increase the efficiency
b. To decrease the efficiency
c. To reduce the work input
d. To increase the work output
Solution: Answer: c. To reduce the work input
Question 3: The Bell Coleman cycle is commonly used in:
a. Steam power plants
b. Refrigeration systems
c. Internal combustion engines
d. Nuclear power plants
Solution: Answer: b. Refrigeration systems
Question 4: The compression and expansion processes in the Bell Coleman cycle are generally:
a. Isothermal
b. Isobaric
c. Adiabatic
d. Isentropic
Solution: Answer: c. Adiabatic
Question 5: In the Bell Coleman cycle, the heat rejection process takes place at:
a. Constant pressure
b. Constant volume
c. Constant temperature
d. Constant entropy
Solution: Answer: a. Constant pressure
Question 6: In the Bell Coleman cycle, the cooling effect is achieved during the process of:
a. Compression
b. Expansion
c. Heat addition
d. Heat rejection
Solution: Answer: b. Expansion
Question 7: The Bell Coleman cycle is a modification of which thermodynamic cycle?
a. Carnot cycle
b. Rankine cycle
c. Otto cycle
d. Brayton cycle
Solution: Answer: d. Brayton cycle
Question 8: The efficiency of the Bell Coleman cycle is increased by:
a. Increasing the pressure ratio
b. Increasing the temperature at which heat is added
c. Decreasing the temperature at which heat is rejected
d. Increasing the specific heat of the working fluid
Solution: Answer: a. Increasing the pressure ratio
Question 9: The Bell Coleman cycle is a:
a. Refrigeration cycle
b. Power cycle
c. Both a and b
d. None of the above
Solution: Answer: a. Refrigeration cycle
Question 10: The process of mixing the cold refrigerant leaving the expansion valve with a portion of the warm refrigerant leaving the compressor is known as:
a. Intercooling
b. Reheat
c. Subcooling
d. Regeneration
Solution: Answer: b. Reheat
Question 11: For a Brayton cycle operating with air as the working fluid, if the compression ratio is 8 and the temperature at the beginning of compression is 300 K, calculate the temperature at the end of compression. Assume γ (specific heat ratio) for air is 1.4.
a. 1200 K
b. 600 K
c. 900 K
d. 1500 K
Solution: Answer: a. 1200 K
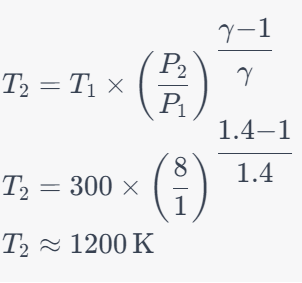
Question 12: In a Brayton cycle, if the turbine inlet temperature is 1000 K, and the temperature at the end of compression is 300 K, calculate the thermal efficiency. Assume γ for air is 1.4.
a. 60%
b. 40%
c. 30%
d. 25%
Solution: Answer: a. 60%

Question 13: For a Brayton cycle, if the compressor work is 400 kJ/kg and the turbine work is 300 kJ/kg, calculate the net work output per unit mass of air.
a. 100 kJ/kg
b. 700 kJ/kg
c. 1000 kJ/kg
d. 200 kJ/kg
Solution: Answer: b. 700 kJ/kg
Net Work Output = 400−300
Net Work Output=100 kJ/kg
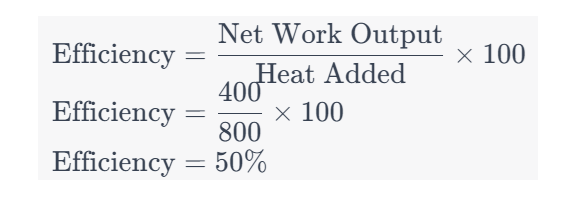
Question 14: If the heat added in a Brayton cycle is 800 kJ/kg and the net work output is 400 kJ/kg, calculate the thermal efficiency.
a. 50%
b. 25%
c. 75%
d. 33.33%
Solution: Answer: a. 50%
Question 15: For a Brayton cycle, if the pressure ratio is 6 and the temperature at the beginning of compression is 300 K, calculate the temperature at the end of compression. Assume γ for air is 1.4.
a. 900 K
b. 1200 K
c. 600 K
d. 1500 K
Solution: Answer: a. 900 K