In this article you will learn about Reversed Carnot Cycle, Processes in Reversed Carnot Cycle, How to Find out COP and Its formula, Limitations of Reversed Carnot Cycle, PV, TS Diagram and Applications, you can also read about What is Boot Strap Air Cooling Refrigeration System?
What is Reversed Carnot Cycle?
It is also known as Ideal Refrigeration cycle, it is working in the reversed order of Carnot Cycle, Carnot cycle having maximum efficiency but in case of reversed Carnot cycle we can get maximum COP of the refrigerating device.
Here we will consider air as refrigerant but in practically we used different refrigerants now a days we are using eco friendly refrigerant which is chlorine free means no ozone depletion.
Processes of Reversed Carnot Cycle-
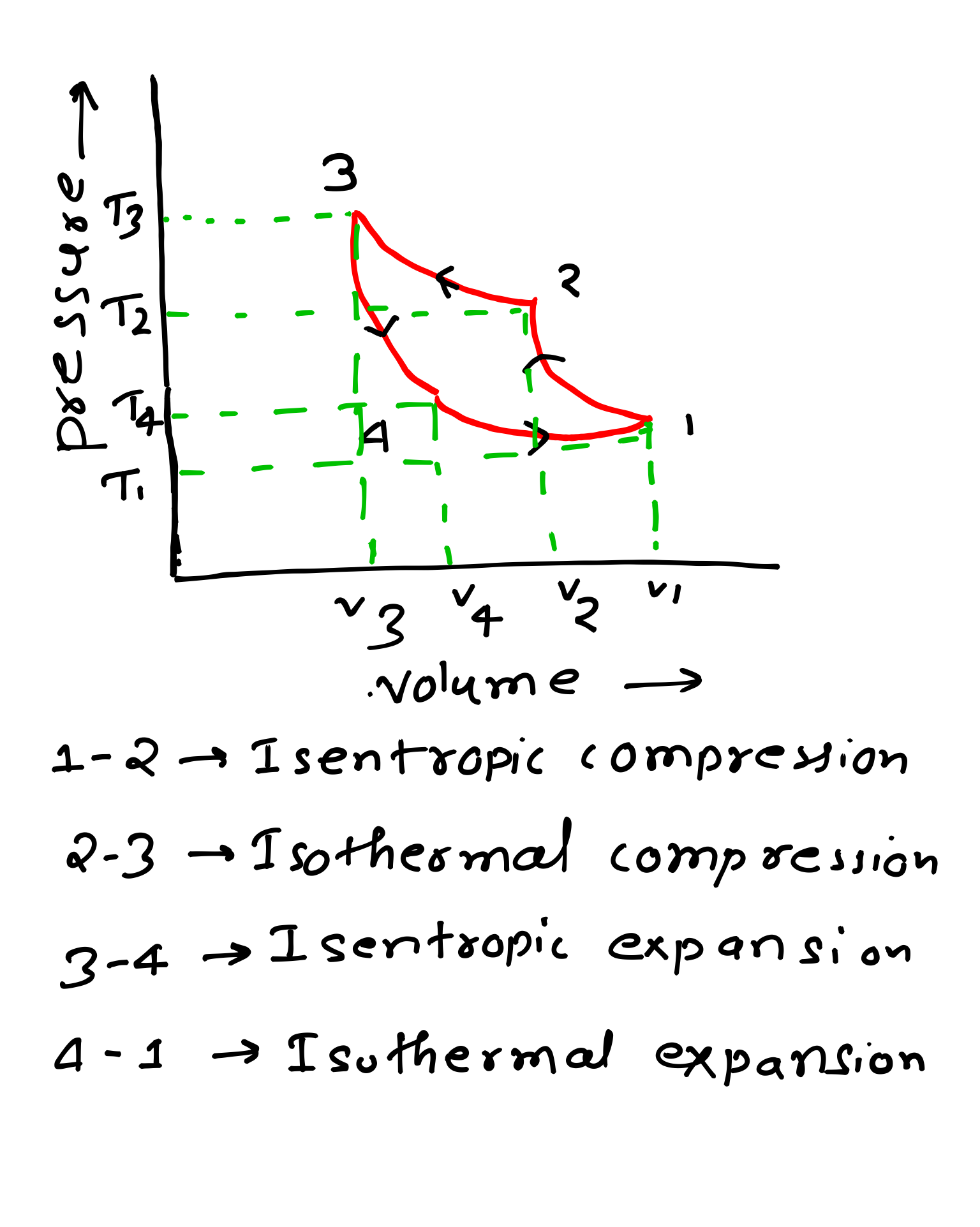
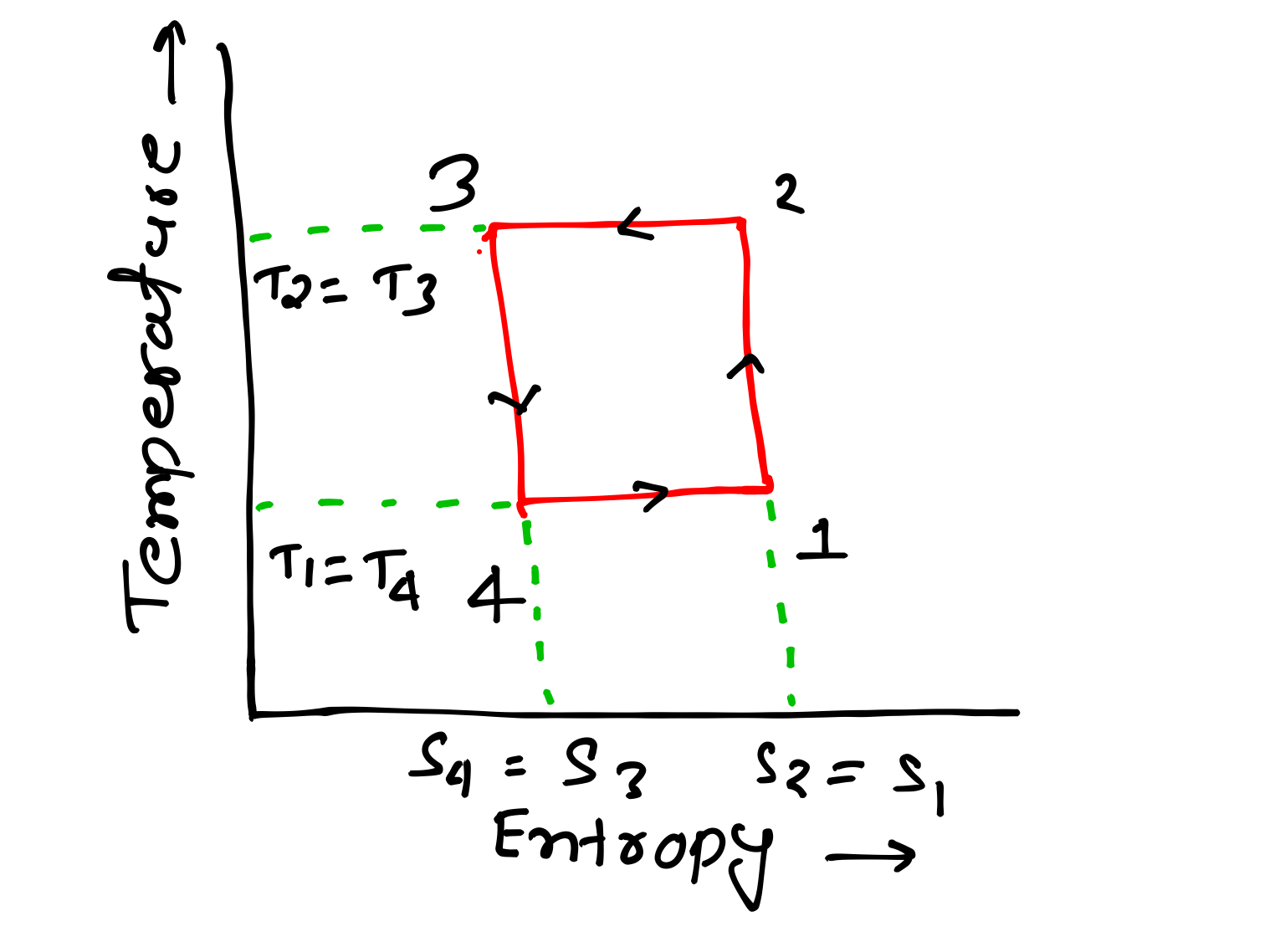
I have shared PV and TS diagram of all these processes below you can refer for getting better understanding about all these four processes.
Isentropic Compression Process-
1-2 Process- It is also known as reversible adiabatic process in this case no heat is absorbed or rejected by the air. In this process as we can see pressure is increasing but specific volume is decreasing that is why this process is a compression process.
Note- Pressure increases, temperature increases but specific volume decreases, in this process entropy is constant.
Mathematically, we can represent it as-
Q1-2 = 0 because no heat absorbed or rejected during this process by air.
Isothermal Compression Process-
2-3 Process- In this process temperature is constant but pressure increases and specific volume decreases.
Mathematically, we can represent it as-
Q2-3 = T3(S2- S3)
Isentropic Expansion Process-
3-4 Process- In this process air is expanded isentropically we can it on PV and TS diagram on the below I have added.
In this process, pressure and temperature decreases but specific volume increases.
Mathematically, we can represent it as-
Q3-4 = 0 because no heat absorbed or rejected during this process by air.
Isothermal Expansion Process-
4-1 Process- In this process air is expanded isothermally that means temperature is remain constant in this process.
In this process pressure decreases but specific volume increases.
Mathematically, we can represent it as-
Q4-1 = T4(S1 – S2) = T4(S2 – S3)
PV and TS Diagram of Reversed Carnot Cycle-
COP of Reversed Carnot Cycle-
Coefficient of performance(COP) of Reversed Carnot Cycle is-
COP = Desire Effect/Work Input = Q4-1/(Q2-3 – Q4-1)
In terms of temperature, COP = T1/(T2- T1)
Limitation of Reversed Carnot Cycle-
- It is depend on temperature of the hot body and cold body as well
- In practical it is impossible to get because in practical we can not get reversible process.
Application of Reversed Carnot Cycle-
- Refrigeration Systems:
- The most common application of the Reversed Carnot Cycle is in refrigeration systems, such as domestic refrigerators, air conditioning units, and industrial refrigeration units.
- In these systems, the cycle is used to absorb heat from the interior of the refrigerated space (cold reservoir) and reject it to the external environment (hot reservoir), thereby maintaining a lower temperature in the system, means where we need cooling effect.
- Air Conditioning:
- Air conditioning systems used the Reversed Carnot Cycle to cool inside home or office by removing heat from the interior and adding it outdoors.
- By controlling the flow of refrigerants by using any external source of work, air conditioning systems can provide comfort cooling in homes, offices, and various commercial buildings.
- Heat Pumps:
- Heat pump systems used the Reversed Carnot Cycle to provide both heating and cooling. They can extract heat from the outdoor environment to warm inside space during colder months and vice-versa during warmer months.
- Heat pumps are energy-efficient solutions for climate control and can be used in domestic, commercial, and industrial applications as well.
- Industrial Processes:
- Some industrial processes require precise temperature control and cooling. The Reversed Carnot Cycle is applied in industries where maintaining specific temperature ranges is very important for product quality or equipment operation.
- We can see as an examples include food processing, pharmaceuticals manufacturing and others.
- Renewable Energy:
- The Reversed Carnot Cycle is used in geothermal heat pump systems, which use the constant temperature of the Earth’s subsurface as a heat source in the winter and a heat sink in the summer.
- This technology allows for efficient heating and cooling in a sustainable as well as environmentally friendly way.
- Cryogenic Applications:
- In scientific and medical fields, the Reversed Carnot Cycle is used in cryogenic applications. It plays very important role in liquefying gases like nitrogen and oxygen for medical and industrial use.
- Cryogenic applications often require extremely low temperatures, that we can get by using specialized refrigeration cycles based on the Reversed Carnot Cycle.
- Aerospace:
- Some spacecraft and satellites use thermodynamic cycles based on the Reversed Carnot Cycle to manage temperature control in space, where there is a vacuum and a need to dissipate or retain heat efficiently so that it will be comfortable for human to be there safely.
FAQ(Frequently Asked Questions)-
What is the reason that the reversed Carnot cycle can’t be practically achieved?
Because all reversible processes are impossible to get practically, but this cycle is used as standard for getting maximum COP from refrigerating device like refrigerator.
What is the Other Name of Reversed Carnot Cycle?
Answer- Other name of Reversed Carnot Cycle is Ideal Refrigeration Cycle.
Reversed Carnot Cycle MCQ’s with Solution-
These MCQ’s you can consider for various exams as model paper like GATE, SSC JE or any other exams like your university as well.
Question 1: A reversed Carnot engine operates between two reservoirs. The hot reservoir has an absolute temperature of 500 K, and the cold reservoir has an absolute temperature of 300 K. Calculate the efficiency of this cycle.
A) 40%
B) 50%
C) 60%
D) 70%
Explanation: The efficiency of a reversed Carnot cycle can be calculated using the formula:
Efficiency=
Where is the absolute temperature at which heat is added, and is the absolute temperature at which heat is rejected.
In this case, and .
Efficiency = 1−300/500= or 40%.
So, the correct answer is (A) 40%.
Question 2: If a reversed Carnot engine has an efficiency of 40% and absorbs 800 J of heat from the hot reservoir, how much work does it produce?
A) 160 J
B) 240 J
C) 320 J
D) 400 J
Explanation: The work produced by a reversed Carnot engine can be calculated using the formula:
Work=Efficiency × Heat Absorbed
In this case, Efficiency = 0.40 and Heat Absorbed = 800 J.
Work = 0.40 × 800 J=.
So, the correct answer is (C) 320 J.
Question 3: A reversed Carnot refrigerator is used and operates between two reservoirs. The hot reservoir has an absolute temperature of 400 K, and the cold reservoir has an absolute temperature of 200 K. Calculate the coefficient of performance (COP) of this refrigerator.
A) 1.0
B) 1.5
C) 2.0
D) 2.5
Explanation: The coefficient of performance (COP) for a reversed Carnot refrigerator can be calculated using the formula:
COP=
Where is the absolute temperature of the hot reservoir, and is the absolute temperature of the cold reservoir.
In this case, and .
COP = 200/(400−200).
So, the correct answer is (A) 1.0.
Question 4: A reversed Carnot engine operates between two reservoirs. The hot reservoir has an absolute temperature of 550 K, and the cold reservoir has an absolute temperature of 350 K. Calculate the efficiency of this cycle.
A) 36.36%
B) 45.45%
C) 54.54%
D) 63.64%
Explanation: The efficiency of a reversed Carnot cycle can be calculated using the formula:
Efficiency=
Where is the absolute temperature at which heat is added, and is the absolute temperature at which heat is rejected.
In this case, and .
Efficiency = 1−350/550≈0.3636 or 36.36%.
So, the correct answer is (A) 36.36%.
Question 5: If a reversed Carnot engine has an efficiency of 35% and absorbs 700 J of heat from the hot reservoir, how much work does it produce?
A) 140 J
B) 210 J
C) 280 J
D) 350 J
Explanation: The work produced by a reversed Carnot engine can be calculated using the formula:
Work=Efficiency × Heat Absorbed
In this case, Efficiency = 0.35 and Heat Absorbed = 700 J.
Work = 0.35×700 J=245 J.
So, the closest answer is (B) 210 J.
Question 6: A reversed Carnot engine operates between two reservoirs. The hot reservoir has an absolute temperature of 500 K, and the cold reservoir has an absolute temperature of 200 K. Calculate the efficiency of this cycle.
A) 60%
B) 70%
C) 75%
D) 80%
Explanation: The efficiency of a reversed Carnot cycle can be calculated using the formula:
Efficiency
Where is the absolute temperature at which heat is added, and is the absolute temperature at which heat is rejected.
In this case, and .
Efficiency = 1−200/500= or 60%.
So, the correct answer is (A) 60%.
Question 7: A reversed Carnot refrigerator operates between two reservoirs at temperatures of 350 K and 250 K. What is the coefficient of performance (COP) of the refrigerator in an SSC JE exam?
A) 1.4
B) 2.0
C) 2.5
D) 3.0
Explanation: The COP can be calculated using the formula:
COP=
Where is the absolute temperature of the hot reservoir, and is the absolute temperature of the cold reservoir.
In this case, and .
COP = 250/(350−2500= .
So, the correct answer is (C) 2.5
Question 8: A reversed Carnot cycle is operated between two reservoirs. The hot reservoir has an absolute temperature of 700 K, and the cold reservoir has an absolute temperature of 400 K. Calculate the efficiency of this cycle.
A) 33.33%
B) 44.44%
C) 55.56%
D) 66.67%
Explanation: The efficiency can be calculated using the formula:
Efficiency=
Where is the absolute temperature at which heat is added, and is the absolute temperature at which heat is rejected.
In this case, and .
Efficiency = 1−400/700≈ 0.4286 or 42.86%.
So, the closest answer is (B) 44.44%.
Question 9: A reversed Carnot engine operates between two reservoirs. The hot reservoir has an absolute temperature of 600 K, and the cold reservoir has an absolute temperature of 300 K. What is the efficiency of this cycle?
A) 20%
B) 40%
C) 50%
D) 60%
Explanation: The efficiency of a reversed Carnot cycle can be calculated using the formula:
Efficiency=
Where is the absolute temperature at which heat is added, and is the absolute temperature at which heat is rejected.
In this case, and .
Efficiency = 1−300/600=, or 50%.
So, the correct answer is (C) 50%.
Question 10: A reversed Carnot refrigerator is used, and it operates between two reservoirs. The hot reservoir has an absolute temperature of 350 K, and the cold reservoir has an absolute temperature of 200 K. Calculate the coefficient of performance (COP) of this refrigerator.
A) 1.25
B) 1.50
C) 1.75
D) 2.00
Explanation: The coefficient of performance (COP) for a reversed Carnot refrigerator can be calculated using the formula:
COP=
Where is the absolute temperature of the hot reservoir, and is the absolute temperature of the cold reservoir.
In this case, and .
COP = 200/(350−200)= 1.33
So, the closest answer is (A) 1.25.