tIt is a cycle this article we will learn about Reversed Carnot Cycle and its definition, diagram, COP, efficiency(does not exist, why? I will share later in this article) and also limitations.
Lets, start with Carnot Cycle?
First lets, learn about Carnot, whose full name is Nicolas Léonard Sadi Carnot, he is French and worked for French army, scientist and also physicist.
What is Carnot Cycle?
It is a theoretical thermodynamic cycle proposed by French physicist Sadi Carnot in 1824.
It is a reversible process that consists of four processes.
Carnot cycle processes-
- Isothermal Expansion: The working substance (e.g. a gas) is in contact with a heat source at a constant high temperature (Th) and is allowed to expand. During this process, the working substance absorbs heat from the heat source and does work on the surroundings.
- Adiabatic Expansion: The working substance is now disconnected from the heat source and allowed to expand adiabatically (without the exchange of heat) until its temperature drops to a lower value (Tc). During this process, the internal energy of the working substance decreases and it does work on the surroundings.
- Isothermal Compression: The working substance is now in contact with a heat sink at a constant low temperature (Tc) and is compressed. During this process, the working substance releases heat to the heat sink and does work on the surroundings.
- Adiabatic Compression: The working substance is now disconnected from the heat sink and is compressed adiabatically (without the exchange of heat) until its temperature returns to the initial high value (Th). During this process, the internal energy of the working substance increases and it does work on the surroundings.
Note that the i is reversible. The cycle can be run in the reverse order and it will produce the same amount of work as in the forward direction.
The Carnot cycle is the most efficient thermodynamic cycle possible, and serves as a benchmark for the performance of real-world heat engines.
The efficiency of a heat engine, measured as the ratio of work output to heat input, is known as the Carnot efficiency, and is dependent on the temperature difference between the heat source and the heat sink.
What is Reversed Carnot Cycle?
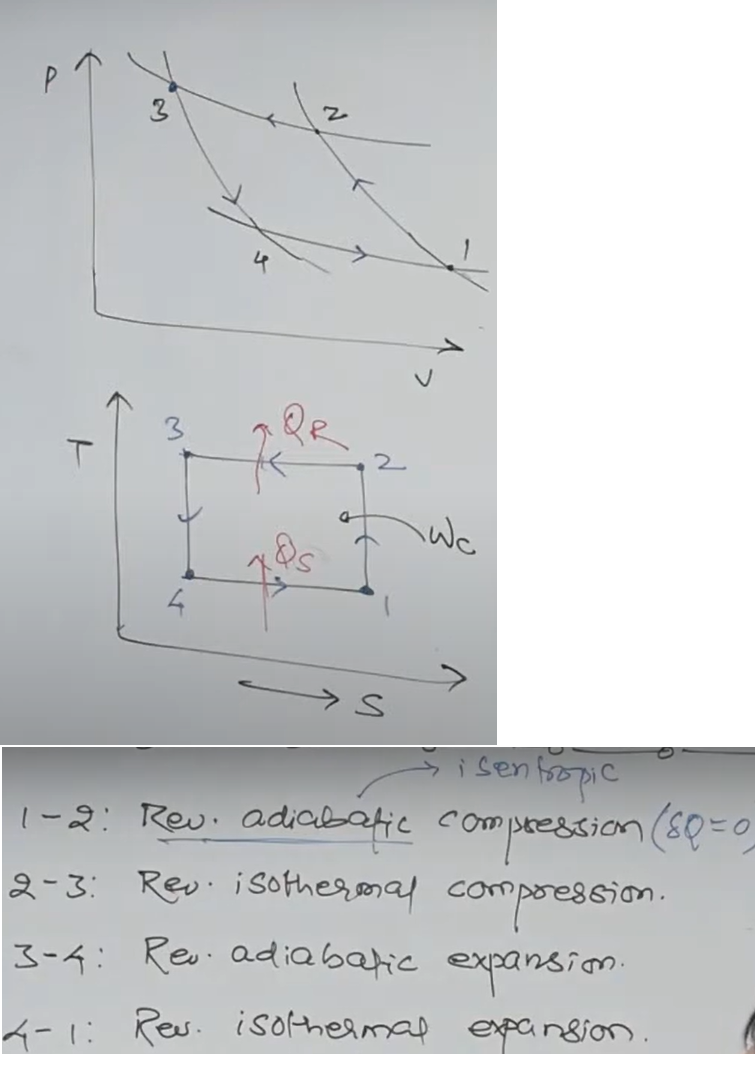 The reversed Carnot cycle is the theoretical cycle in which a heat engine operates in the reverse direction, functioning as a refrigerator or heat pump.
The reversed Carnot cycle is the theoretical cycle in which a heat engine operates in the reverse direction, functioning as a refrigerator or heat pump.
It is the inverse of the Carnot cycle, which is an idealized thermodynamic cycle that describes the maximum efficiency of a heat engine.
In the reversed Carnot cycle, the working fluid is cooled while absorbing heat from the cold reservoir, and then rejected the absorbed heat to the hot reservoir.
Maximum coefficient of performance (COP) of a refrigerator or heat pump is given by the ratio of the heat absorbed by the working fluid from the cold reservoir to the work done on the working fluid.
COP of RCC-
COP of Refrigerator = Refrigeration Effect/Work Input = Q2/(Q1-Q2) = TL/(TH – TL))
COP of Heat Pump = 1 + COP of Refrigerator
This is known as the reversed Carnot efficiency.
Why RCC is not Possible Practically?
Reversed Carnot cycle is not possible to get practically because it is needed a perfect heat sink, which is able to absorb an infinite amount of heat without any temperature increase.
In practice, all heat sinks have a finite heat capacity, so they will experience a temperature increase when absorbing heat.
This means that the cold reservoir in a real refrigerator or heat pump will not be able to reach absolute zero, and the coefficient of performance will be limited by the temperature of the cold reservoir.
Additionally, the processes in a real refrigerator or heat pump are not reversible and will always involve some internal irreversibilities, such as friction and turbulence, that will further decrease the efficiency of the cycle.
Keep Reading- Catalytic Converter and Types of Catalytic Converter
Efficiency of Reversed Carnot Cycle?
Carnot cycle having maximum efficiency but this is not having efficiency it is having COP that is coefficient of performance.
Is a Carnot Cycle Always Reversible?
A Carnot cycle is a theoretical thermodynamic cycle that is reversible and isothermal.
In reality, no heat engine can operate with 100% efficiency, so a Carnot cycle cannot be achieved in practice.
However, the this cycle serves as a theoretical upper limit for the efficiency of a heat engine, and is often used as a benchmark for comparing the performance of real-world heat engines.
Keep Reading-Carburetor and Types of Carburetor
Why Reversible Process is not Possible?
Reversible processes are not possible in practice because they require an infinite amount of time and an absence of any friction or other types of energy dissipation.
In real-world systems, there will always be some amount of friction or other forms of energy dissipation that will prevent a process from being reversible.
Additionally, real-world processes are often limited by the availability of resources, such as the temperature of a heat reservoir, which can prevent them from being reversible.
Reversibility is the ideal case, in real world, many processes are irreversible due to the presence of friction, dissipative forces, and other forms of energy loss.
For example, in a piston-cylinder system, the movement of the piston creates friction with the cylinder wall, which results in energy loss in the form of heat.
Additionally, the pressure and temperature of the working fluid may change during the process, which further adds to the irreversibility of the process.
Keep Reading- What is the Difference between an IC and an SI Engine?
Advantages and Disadvantages of Carnot Cycle-
Advantages of the Carnot cycle:
- High efficiency: This cycle is the most efficient thermodynamic cycle, as it has the highest theoretical efficiency of any heat engine.
- Universality: This cycle can be applied to any heat engine, regardless of the working substance used.
- Reversibility: This cycle is reversible, meaning that it can be run in either direction and the same amount of heat will be absorbed or rejected.
Disadvantages of the Carnot cycle:
- Idealized: It is a theoretical ideal and cannot be achieved in practice because it requires infinitely slow and reversible processes.
- Low temperature limits: This cycle requires a low-temperature heat reservoir, which may not be available in certain applications.
- High pressure and temperature differentials: This cycle requires a high-temperature heat reservoir and a low-temperature heat sink, which can be difficult to achieve in some applications.
- High cost : This cycle requires high-quality insulation and other components to minimize heat loss, which can be costly.
In summary, It is is an idealized cycle that is not possible to achieve in practice, but serves as a theoretical upper limit for the efficiency of a heat engine.
It has high efficiency, universality, and reversibility but it also has low temperature limits, high pressure and temperature differentials and high cost.
Keep Reading- What is Scavenging in 2 Stroke & 4 Strokes Engines
Reversed Carnot Cycle is also Known as-
It is also known as a Carnot refrigerator or a Carnot heat pump. It operates in the reverse direction of a Carnot heat engine, where it absorbs heat from a low-temperature reservoir and reject heat to a high-temperature reservoir.
The goal of a Carnot refrigerator is to cool a space or object by removing heat from it and rejecting it to a higher-temperature heat sink.
The coefficient of performance (COP) of a Carnot refrigerator is the ratio of the heat removed from the cold reservoir to the work input.
And the maximum COP is limited by the Carnot cycle efficiency.
Carnot Cycle vs Reversed Carnot Cycle-
Carnot Cycle:-
| Step | Process | Heat Added | Heat Rejected |
|---|---|---|---|
| 1 | Isothermal Expansion at High Temperature (Th) | Qh | 0 |
| 2 | Adiabatic Expansion | 0 | 0 |
| 3 | Isothermal Compression at Low Temperature (Tc) | 0 | Qc |
| 4 | Adiabatic Compression | 0 | 0 |
Reversed Carnot Cycle:-
| Step | Process | Heat Added | Heat Rejected |
|---|---|---|---|
| 1 | Isothermal Compression at High Temperature (Th) | 0 | Qh |
| 2 | Adiabatic Compression | 0 | 0 |
| 3 | Isothermal Expansion at Low Temperature (Tc) | Qc | 0 |
| 4 | Adiabatic Expansion | 0 | 0 |
Note: Qh and Qc are heat transferred in the process.
Limitations of Reversed Carnot Cycle-
The reversed Carnot cycle, also known as the reversed Carnot heat engine, has several limitations:
- Efficiency: The efficiency of the this cycle is less than that of the Carnot cycle because it operates in the opposite direction.
- Practicality: This cycle is not practical as it requires a source of heat at a high temperature and a sink at a low temperature, which is difficult to find in nature.
- Complexity: This cycle is more complex than the Carnot cycle, as it requires additional components to reverse the direction of heat flow.
- Mechanical Work: In this, mechanical work is produced in the direction opposite to the usual one. This may require additional components and increase complexity.
- Heat Pumping : it is more efficient as a heat pump than reversed Carnot cycle.
- Second Law of Thermodynamics: The second law of thermodynamics states that it is impossible to have a heat engine that operates at an efficiency of 100%. The reversed Carnot cycle is no exception and must still abide by this law.